Validation of an anti-α-Gal IgE fluoroenzyme-immunoassay for the screening of patients at risk of severe anaphylaxis to cetuximab, BMC Cancer
Por um escritor misterioso
Last updated 19 junho 2024
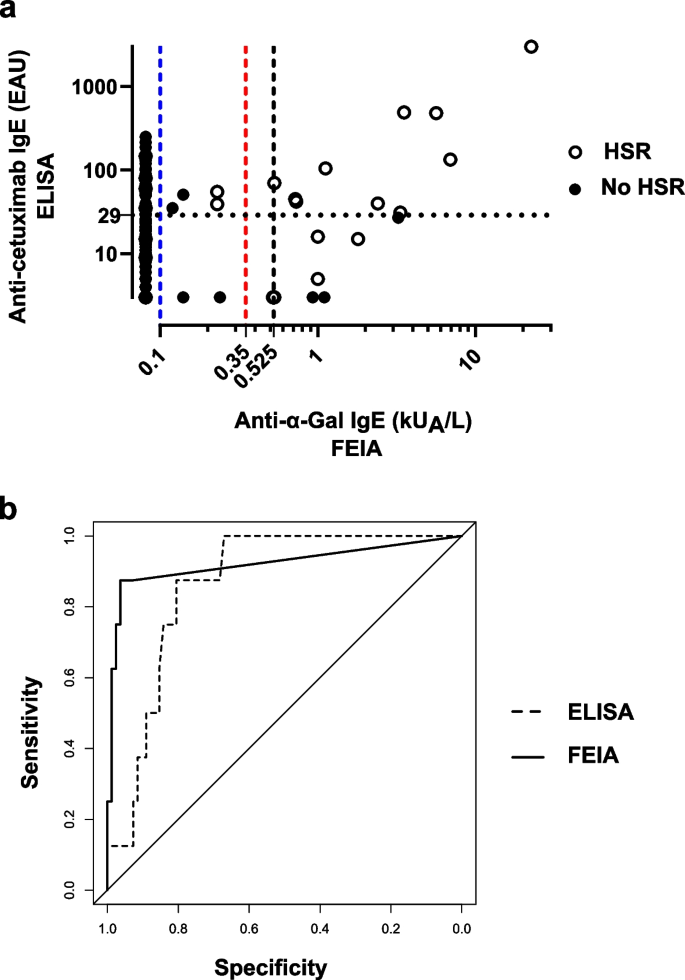
Background The link between immediate hypersensitivity reactions (HSR) following the first cetuximab infusion and the IgE sensitization against anti-galactose-α-1,3-galactose (α-Gal) is now well-established. An automated Fluoroenzyme-Immunoassay (FEIA) is available and may facilitate the screening of patients with anti-α-Gal IgE before treatment. Methods This study aimed to evaluate its performances as compared to a previously validated anti-cetuximab IgE ELISA, using 185 samples from two previously studied cohorts. Results Despite 21.1% of discrepancies between the two techniques, FEIA discriminated better positive patients and similarly negative ones with a ≥ 0.525 kUA/L threshold. Sensitivity was 87.5% for both tests, specificity was better for FEIA (96.3% vs ELISA: 82.1%). FEIA had a higher positive likelihood ratio (23.9 vs ELISA: 4.89) and a similar negative likelihood ratio (0.13 vs ELISA: 0.15). In our population, the risk of severe HSR following a positive test was higher with FEIA (56.7% vs ELISA: 19.6%) and similar following a negative test (0.7% vs ELISA: 0.8%). Conclusion Although the predictive value of the IgE screening before cetuximab infusion remains discussed, this automated commercial test can identify high-risk patients and is suitable for routine use in laboratories. It could help avoiding cetuximab-induced HSR by a systematic anti-α-Gal IgE screening before treatment.

PDF) Validation of an anti-α-Gal IgE fluoroenzyme-immunoassay for the screening of patients at risk of severe anaphylaxis to cetuximab

Suppression of IgE-mediated anaphylaxis and food allergy with monovalent anti-FcεRIα mAbs - ScienceDirect

Forest plot and SROC curve. Download Scientific Diagram

Patient characteristics (n = 92)

Gautier Petit's research works Centre Hospitalier Universitaire de Caen, Caen (CHU Caen) and other places

Risk factors associated with hypersensitivity reactions to cetuximab: anti-cetuximab IgE detection as screening test
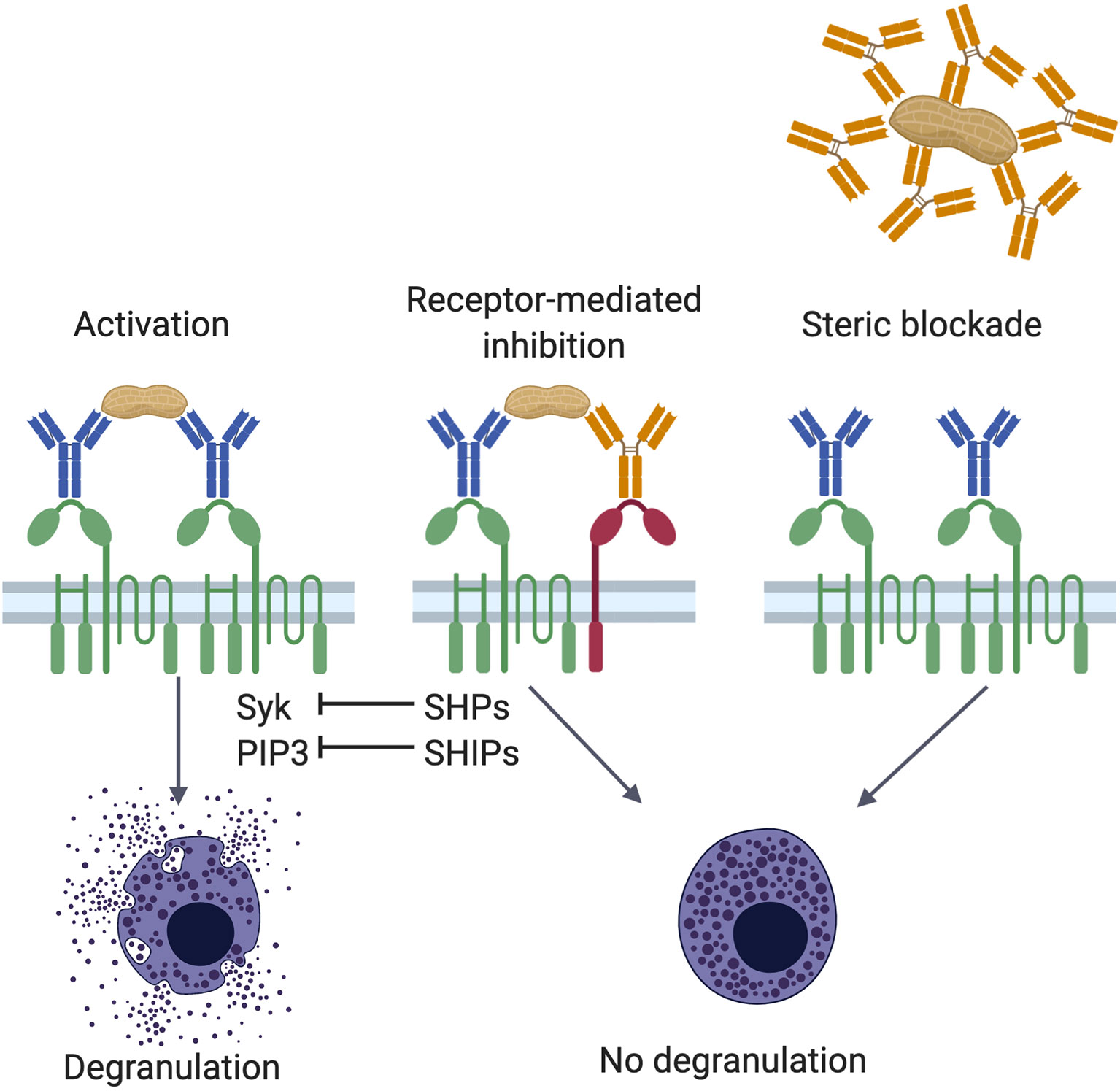
Frontiers IgE and IgG Antibodies as Regulators of Mast Cell and Basophil Functions in Food Allergy
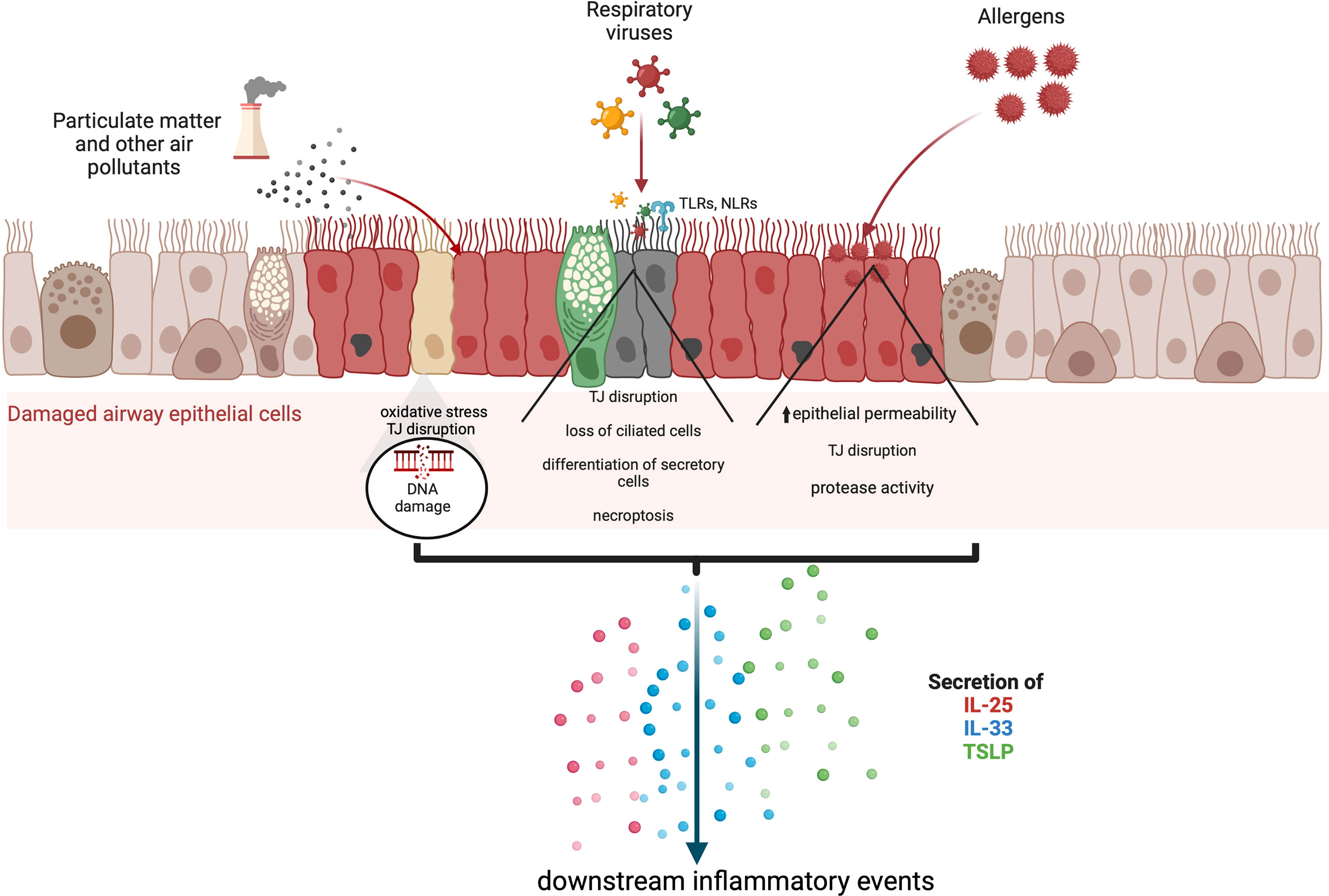
Frontiers Epithelial cell alarmin cytokines: Frontline mediators of the asthma inflammatory response
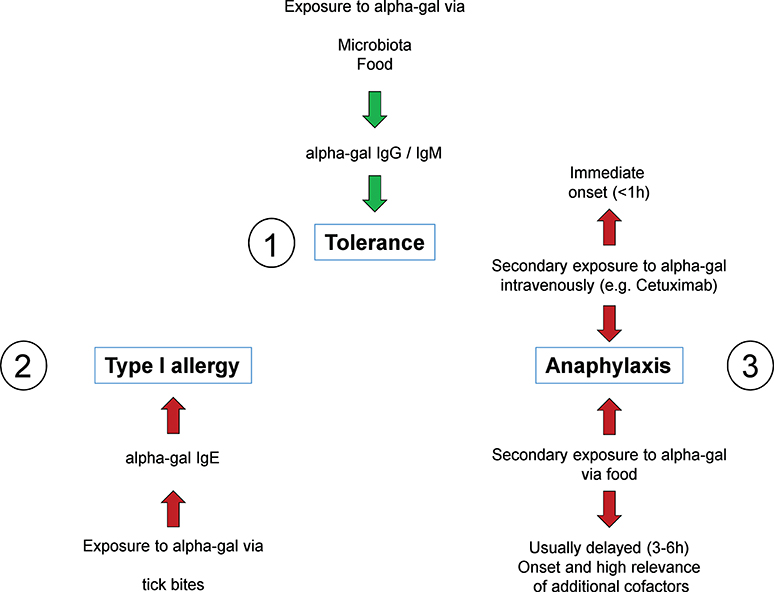
Frontiers The History of Carbohydrates in Type I Allergy

The basophil activation test differentiates between patients with alpha-gal syndrome and asymptomatic alpha-gal sensitization - ScienceDirect

Anti-IgE as a mast cell–stabilizing therapeutic agent - ScienceDirect
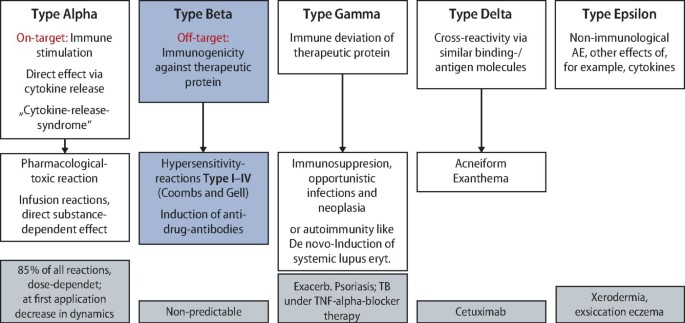
Hypersensitivity reactions to biologics (part II): classifications and current diagnostic and treatment approaches*
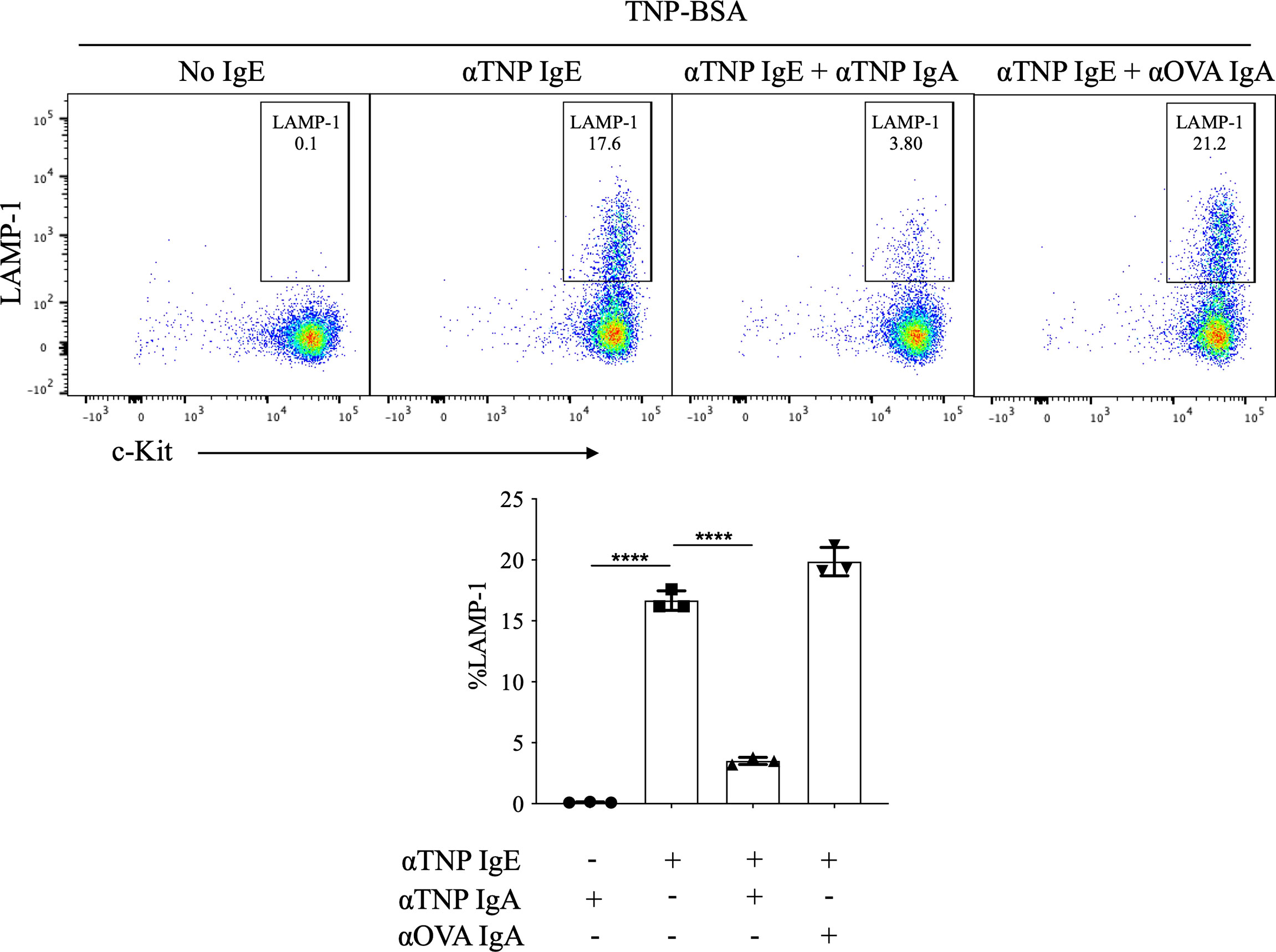
Frontiers Allergen-Specific IgA Antibodies Block IgE-Mediated Activation of Mast Cells and Basophils
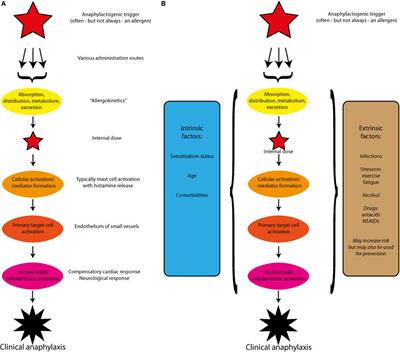
Frontiers Beyond IgE—When Do IgE-Crosslinking and Effector Cell Activation Lead to Clinical Anaphylaxis?
Forest plot and SROC curve. Download Scientific Diagram
Recomendado para você
-
Comidas Feia - Se nóis legendar estraga19 junho 2024
-
 Oblee Marketplace Caixa para doces Castelo Frozen19 junho 2024
Oblee Marketplace Caixa para doces Castelo Frozen19 junho 2024 -
:quality(70)/cloudfront-us-east-1.images.arcpublishing.com/lanacionpy/KHGAXI2CGZH5BABZ67WHZUHBNY.jpg) La Nación / Reacción de pequeña cumpleañera a torta personalizada de “Frozen” se hizo viral19 junho 2024
La Nación / Reacción de pequeña cumpleañera a torta personalizada de “Frozen” se hizo viral19 junho 2024 -
 Frozen ganhará live-action na Disney, diz site19 junho 2024
Frozen ganhará live-action na Disney, diz site19 junho 2024 -
 elsa weird cake|TikTok Search19 junho 2024
elsa weird cake|TikTok Search19 junho 2024 -
 Kit 2 Frozen Boneca Princesa Elsa E Ana Disney Envio 24h19 junho 2024
Kit 2 Frozen Boneca Princesa Elsa E Ana Disney Envio 24h19 junho 2024 -
 1v3 Insane Bots TOWER ISLAND (Lost Temple) : r/WC319 junho 2024
1v3 Insane Bots TOWER ISLAND (Lost Temple) : r/WC319 junho 2024 -
 Dos desenhos animados Mulher Feia Silicone Mini Beads, Food-Grade Mastigar Dentes Bead, DIY Cadeia Mamilo, Acessórios Jóias, Novo, 10Pcs - AliExpress19 junho 2024
Dos desenhos animados Mulher Feia Silicone Mini Beads, Food-Grade Mastigar Dentes Bead, DIY Cadeia Mamilo, Acessórios Jóias, Novo, 10Pcs - AliExpress19 junho 2024 -
 Caixa Pirâmide Robin Hood19 junho 2024
Caixa Pirâmide Robin Hood19 junho 2024 -
 Hedy Hopper - casper's scare school Photo (37693738) - Fanpop19 junho 2024
Hedy Hopper - casper's scare school Photo (37693738) - Fanpop19 junho 2024
você pode gostar
-
 Cesario (@pequenocesarII) / X19 junho 2024
Cesario (@pequenocesarII) / X19 junho 2024 -
 Neal Caffrey Suits: Dress Like the White Collar Star19 junho 2024
Neal Caffrey Suits: Dress Like the White Collar Star19 junho 2024 -
 Isaac on X: UPDATE #6 IS HERE! Use code “builderman” for 5k subs! 💥 World 2 is now complete, with 3 new stages! ⭐ New tiers of characters! 📷 New cameras! 💙19 junho 2024
Isaac on X: UPDATE #6 IS HERE! Use code “builderman” for 5k subs! 💥 World 2 is now complete, with 3 new stages! ⭐ New tiers of characters! 📷 New cameras! 💙19 junho 2024 -
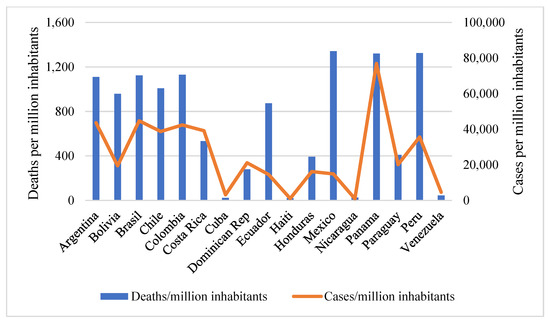 IJERPH, Free Full-Text19 junho 2024
IJERPH, Free Full-Text19 junho 2024 -
 TheMetalBarn19 junho 2024
TheMetalBarn19 junho 2024 -
 Pikachutwo pokemon Mewtwo contraataca by Jorge5H on DeviantArt19 junho 2024
Pikachutwo pokemon Mewtwo contraataca by Jorge5H on DeviantArt19 junho 2024 -
 What would you do if this actually happened? : r/roblox19 junho 2024
What would you do if this actually happened? : r/roblox19 junho 2024 -
 Spaceman (2023) Adam Sandler, Carey Mulligan, Paul Dano, Netflix19 junho 2024
Spaceman (2023) Adam Sandler, Carey Mulligan, Paul Dano, Netflix19 junho 2024 -
 Street Fighter V Vega Street Fighter Alpha 3 Sprite Pixel art, Street Fighter 2, hand, shading png19 junho 2024
Street Fighter V Vega Street Fighter Alpha 3 Sprite Pixel art, Street Fighter 2, hand, shading png19 junho 2024 -
 Zapdos de Galar - Pokémon GO + BRINDE - Pokemon GO - GGMAX19 junho 2024
Zapdos de Galar - Pokémon GO + BRINDE - Pokemon GO - GGMAX19 junho 2024
